UK & EU Commercial launch of SpydrBlade™ Flex
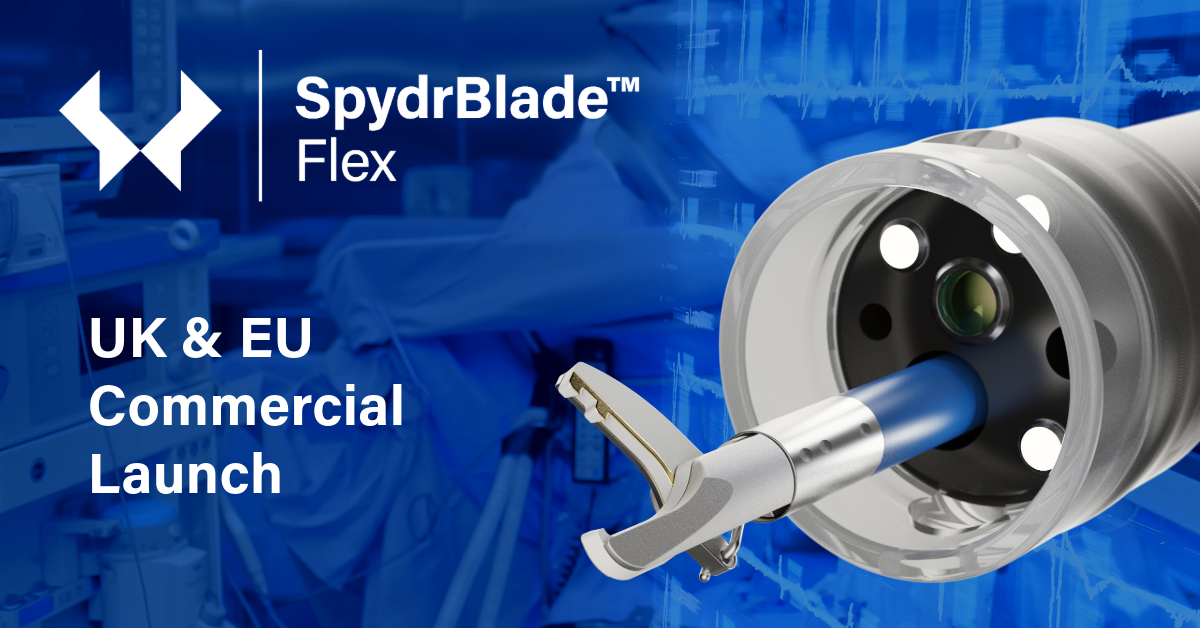
A world Leading NHS Endoscopy unit first commercial customer of SpydrBlade Flex for Lower GI colorectal resections
Creo Medical Group plc (AIM: CREO), the medical device company focused on the emerging field of minimally invasive surgical endoscopy for pre-cancer and cancer patients, announces the commercial launch and first customer of its SpydrBlade Flex1 technology, a unique multi-modal endoscopic device designed for precision and adaptability in endoscopic procedures, in the UK and Europe.
Following extensive pre-launch global clinical activity, this first customer marks the UK & EU commercial launch of SpydrBlade Flex, and is being pioneered in St Mark’s Hospital in NW London, one of the UK’s leading Endoscopy Units and recognised as a world centre of excellence by the WEO (World Endoscopy Organisation). St Mark’s Hospital is also an established and regular user of Creo’s Speedboat UltraSlim.
Creo will be ramping up the full commercial launch via its core sales channels in the UK and the EU as well as other international territories, including the US, once local regulatory approvals have been received.
SpydrBlade Flex will be showcased amongst the latest endoscopy technology at the upcoming ESGE (European Society of Gastrointestinal Endoscopy) Days 2025 conference in Barcelona in April.
Craig Gulliford, Chief Executive Officer of Creo, said:
"We are delighted to announce the commercial launch of our SpydrBlade Flex product in the UK and across Europe and it is fitting that the first commercial customer is such a recognised centre as St Marks. SpydrBlade really is one of the most advanced surgical tools and again, Creo have pioneered the introduction of this technology into the tiny footprint of a flexible endoscopic instrument. Speedboat UltraSlim alongside SpydrBlade Flex will enhance the care of patients with a wide range of conditions ranging from cancers to swallowing disorders, as well as their increasing use in the rapidly growing treatment of Barriatric conditions.
“Both products will continue to help move the point of care from Surgery and the operating theatre to the endoscopy room allowing us to further reduce surgical waiting lists not only in the NHS, but globally. We expect to see further commercial traction as more people adopt our new technology, and following further regulatory approvals, including the FDA clearance to allow our commercial launch in the US.”
RNS released on 20 March 2025
Above: The SpydrBlade Flex “the most versatile dissection tool in flexible endoscopy”. Notes: 1 - https://www.creomedical.com/en/kamaptive-technology/spydrblade-flex
Posted 20/03/2025
For press enquiries please contact media@creomedical.com. For all other enquiries please visit our Contact page.

